 Video Courses
Video Courses Video Courses
Video Courses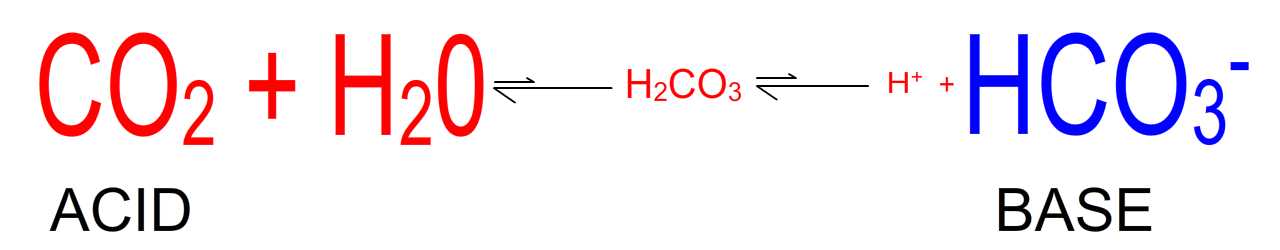
Figure 1. The bicarbonate buffering system in the ECF
1. CO2 is an acid. As shown in the figure above, CO2 when dissolved in water generates carbonic acid and will, therefore, acidify any solution.
2. CO2 is a gas. Its level in the body is controlled by excretion in exhaled air. It is, therefore, referred to as 'respiratory acid'. Disturbances in extracellular fluid (ECF) pH secondary to diseases which alter the level of CO2 in the ECF are referred to as primary respiratory pH disturbances.
3. Bicarbonate is a base. Bicarbonate is a metabolite produced in several metabolic processes. The level of bicarbonate in the ECF is controlled by a balance between its rate of production in the body and its rate of excretion in the kidney. It is referred to as 'metabolic base'. Disturbances in ECF pH secondary to diseases which alter bicarbonate levels are referred to as primary metabolic pH disturbances.
4. Think of acid-base homeostasis as a balance between a respiratory acid (CO2) and an opposing metabolic base (HCO3-).
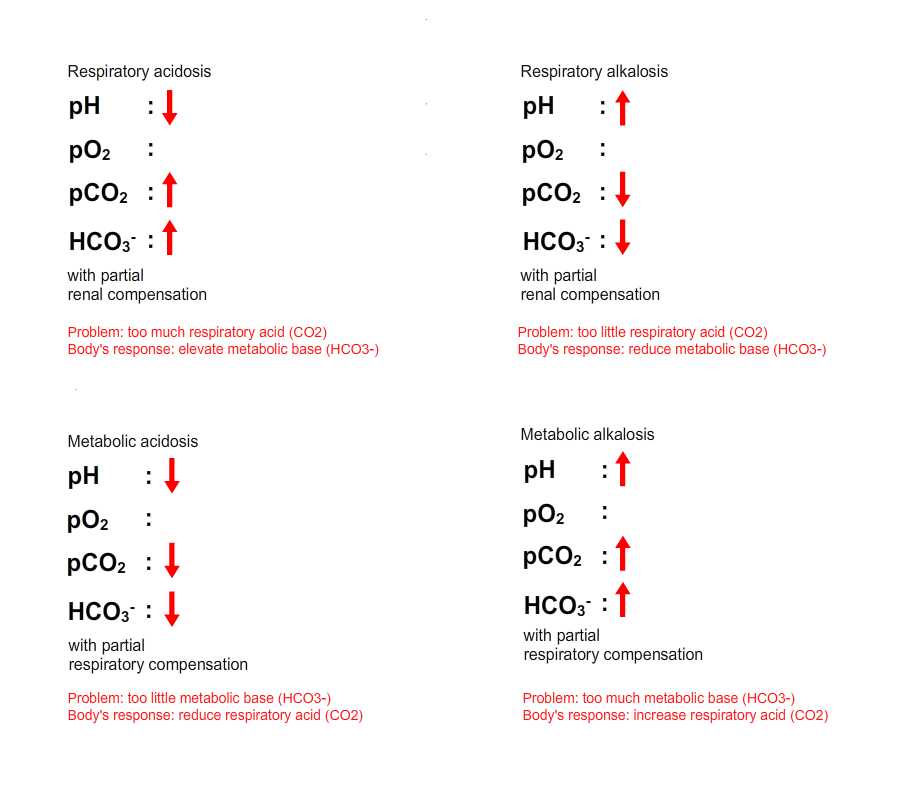
5. A disease process which elevates respiratory acid (CO2) levels in ECF results in a primary respiratory acidosis. A disease process which lowers respiratory acid (CO2) levels in the ECF results in a primary respiratory alkalosis.
6. A disease process which lowers metabolic base (HCO3-) levels in the ECF results in a primary metabolic acidosis. A disease process which elevates metabolic base (HCO3-) levels in the ECF results in a primary metabolic alkalosis.
7. The body can compensate for the change in ECF pH by adjusting the component of the bicarbonate buffering system it still controls. These secondary changes in the bicarbonate buffering system are termed compensation. How does this work?
8. In the presence of a primary metabolic pH disturbance, predictable compensatory changes occur in CO2 levels which oppose the disease-induced change in pH. This is termed respiratory compensation. In the presence of a metabolic acidosis the body will try and get rid of respiratory acid (CO2) in an attempt to bring the pH back towards normal. While in the presence of a metabolic alkalosis the body will try and retain respiratory acid (CO2).
9. In contrast in a patient with a primary respiratory pH disturbance, predictable compensatory changes occur in HCO3- levels opposing the disease-induced change in pH. As this change in bicarbonate levels is mediated by the kidney, this is termed renal compensation. So, in the presence of a respiratory acidosis the body will raise the concentration of metabolic base (HCO3-) in an attempt to bring the pH back towards normal. While in the presence of a respiratory alkalosis the body will decrease the level of metabolic base (HCO3-).
10. In most pH disturbances, compensatory mechanisms move the pH towards the normal range but usually do not succeed in returning it to the normal range. Compensation in these scenarios is said to be partial. This actually helps us to interpret blood gas abnormalities.
Considering the 10 points above, we can easily predict the four patterns of pH disturbance on the ABG
DisclaimerPrivacy PolicyTerms of UseData Deletion© Acadoodle 2026